

This gives us 4 degenerate orbitals, meaning orbitals that have the same amount of energy. We take that s orbital containing 2 electrons and give it a partial energy boost.Īt the same time, we rob a bit of the p orbital energy. Here is how I like to think of hybridization. So what do we do, if we can’t follow the Aufbau Principle?Įnter hybridization! How does hybridization occur? This too is covered in my Electron Configuration videos. This can’t happen though, because the Aufbau Principle says that electrons must fill atomic orbitals from lowest to highest energy. If we can find a way to move ONE of the paired s electrons into the empty p orbital, we’d get something like this. Which isn’t very helpful if we’re trying to build complex macromolecules. With its current configuration, carbon can only form 2 bonds,
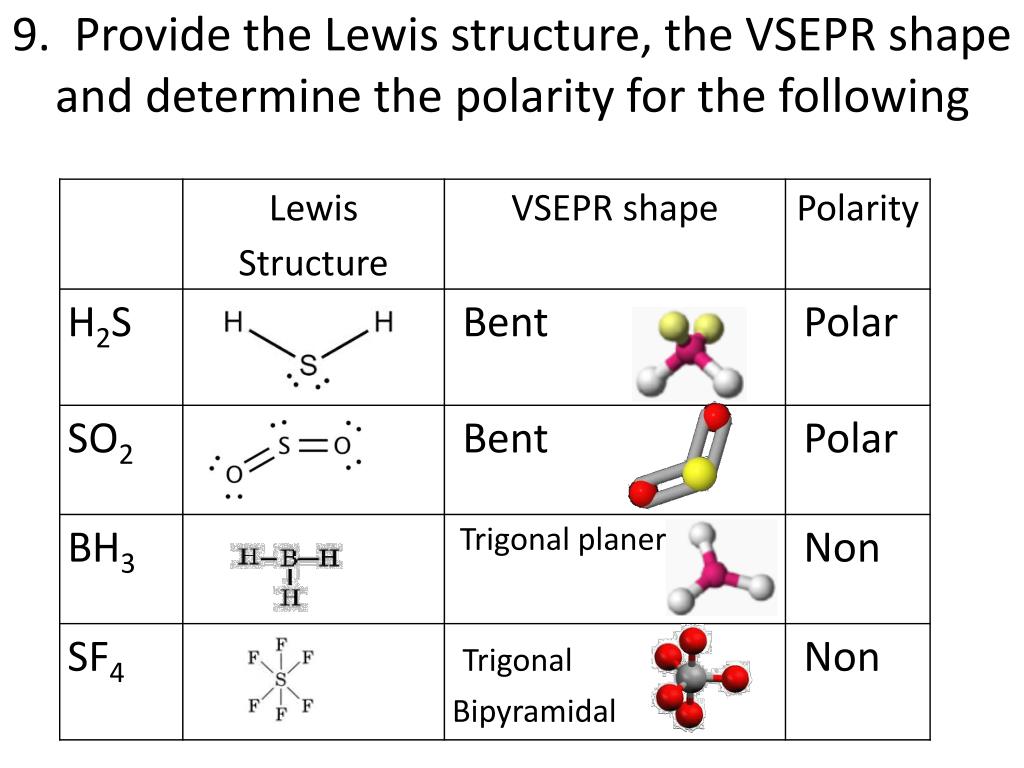
An empty p orbital, lacking the electron to initiate a bond.2 p orbitals, each with a single unpaired electron capable of forming ONE bond.The 2s electrons in carbon are already paired and thus unwilling to accept new incoming electrons in a covalent bond. There are not 4 empty spaces waiting to be filled… YET ! Why? Because carbon is capable of making 4 bonds.Īnd if any of those other atoms are also carbon, we have the potential to build up a giant molecular structure such as ATP, drawn below, a source of energy and genetic building material within cells.Ī review of carbon’s electron configuration shows us that carbon has a total of 6 electrons, with only 4 electrons in its valence shell. Proteins, amino acids, nucleic acids– they all have carbon at the center. It’s no coincidence that carbon is the central atom in all of our body’s macromolecules. Great for adding another hydrogen, not so great for building a large complex molecule. This leaves an opening for one single bond to form. It requires just one more electron to be full. It has a single electron in the 1s orbital. Let’s take a quick detour to review electron configuration with a focus on valence electrons, as they are the ones that actually participate in the bond.įeeling rusty? Click to review my Electron Configuration + Shortcut videos. In order to create a covalent bond (video), each participating atom must have an orbital ‘opening’ (think: an empty space) to receive and interact with the other atom's electrons. With covalent bonds being the strongest and most prevalent. Simply put, molecules are made up of connected atoms,Ītoms are connected through different types of bonds, Molecules are everywhere! How do they form? In addition to undergrad organic chemistry, this topic is critical for exams like the MCAT, GAMSAT, DAT and more. Sp³ d and sp³ d² Hybridization – An Overview.Sp Hybridization, Bond Angle and Geometry.Sp² Hybridization, Bond Angle and Geometry (includes video).
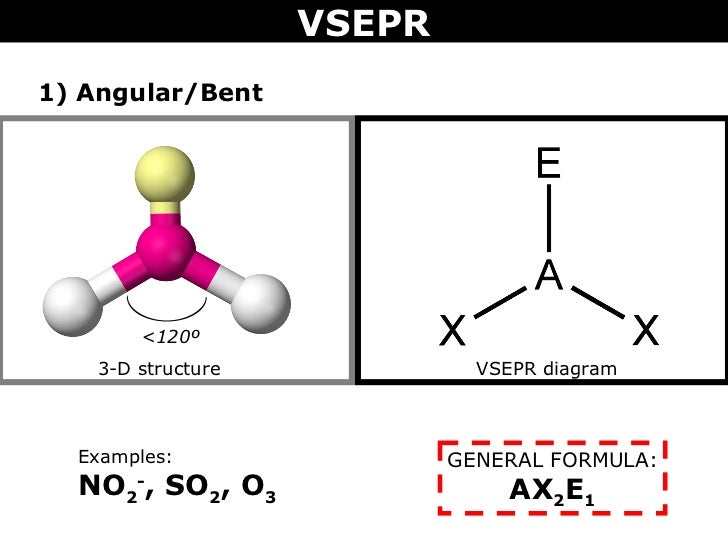
Sp³ Hybridization, Bond Angle and Geometry (includes video).In this article, we’ll cover the following: But it wasn’t until I started thinking of it in a different way, as I’ll explain below, that I finally and truly understood. When I took general chemistry, I simply memorized a chart of geometries and bond angles, and I kinda/sorta understood what was going on. Sp³, sp² and sp hybridization, or the mixing of s and p orbitals which allows us to create sigma and pi bonds, is a topic we usually think we understand, only to get confused when it reappears in organic chemistry molecules and reactions.


 0 kommentar(er)
0 kommentar(er)
